Polyatomic Ions
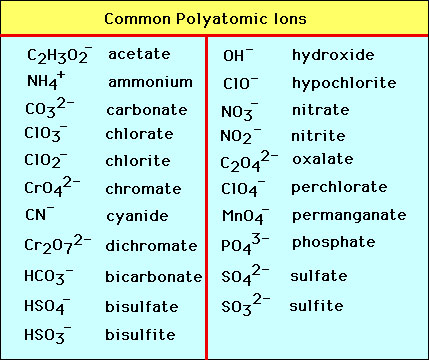
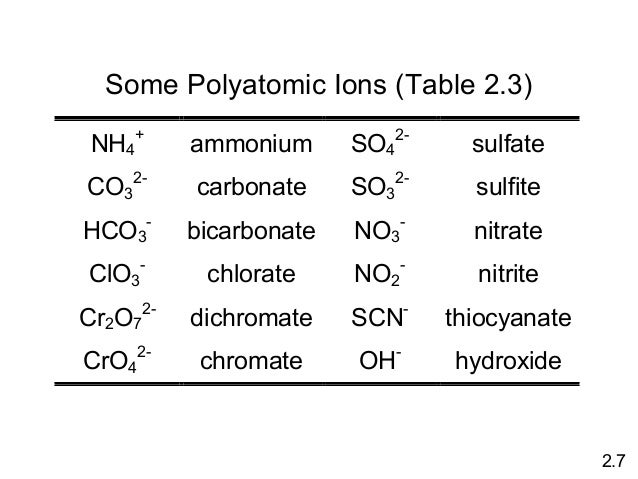
Notice how the four polyatomic-ate ions in the center square (phosphate, arsenate, sulfate, and selenate) all have four oxygen atoms, while the polyatomic -ate ions on the outside all have three oxygen atoms. As you start from right-hand side, the first column of polyatomic -ate ions (chlorate, bromate, iodate) all have a 1- charge. Common Polyatomic Ions Name(s) Formula Name(s) Formula ammonium NH4 + acetate CH3COO-C2H3O2-bromate BrO3-carbonate CO3 2-chlorate ClO3-chlorite ClO2-chromate CrO4 2-cyanide CN-dichromate Cr2O7 2-hydrogen carbonate bicarbonate HCO3-hydrogen sulfate bisulfate HSO4-hydrogen phosphate biphosphate HPO4 2-hydroxide OH. Polyatomic ions are charged groups of atoms. An example is ammonium ion, NH 4 +.It has five atoms (one nitrogen and four hydrogens) that share a charge of +1. The polyatomic ions remain intact, and parentheses may be required when using subscripts. The dotted line around each polyatomic ion in Model 1 shows that the group of atoms has a charge. The charge is not on any one atom, but rather on the group of atoms as a whole. Based on your knowledge of monatomic ions, propose an explanation for the net charge on a polyatomic ion. Polyatomic ions with a positive 1 charge do occur, but the main one you'll encounter and need to know is the ammonium ion. Remember, because it is a cation, when it reacts and forms a compound, it is cited first in the chemical formula. Ammonium - NH 4+ Polyatomic Ion Charge = -1.
Key Questions
Polyatomic Atoms On Bohr Models
The same reason monatomic ions have a charge - due to an imbalance between the number of protons and electrons. 🔴 circles for mac.
Polyatomic ions are composed of two or more covalently bonded atoms - similar to that of a neutral molecule. However, as the name implies, this group of covalently bonded atoms has a difference in the number of electrons v protons. So in the formation of the cyanide ion, for example, CN-, carbon and nitrogen bond together through the formation of a carbon-nitrogen triple bond.
However, if we draw the appropriate structure, you will see that in order for the carbon and nitrogen to achieve stable electron configurations, they will need one more electron than the two of them have collectively - so they obtain an additional electron from a metal donor.
This video may help:
Poly atomic ions are covalent compounds that have an overall charge and therefore are held together through the electrostatic attraction of ionic bonding to positively charged ions called cations.
With the exception of ammonium,
#NH_4^+# , these ions carry a negativeExamples include sulfate,
#SO_4^-2# , nitrate,#NO_3^-# , and phosphate,#PO_4^-3# .If we consider nitrate it will form one ionic bond with cation , such as sodium that has + one charge. The formula is
#NaNO_3# If nitrate bonds with calcium, two nitrate ions each with one calcium ion,
#Ca(NO_3)_2# If nitrate bonds with aluminum which has a plus three charge, three nitrates are required to create an ionic compound,
#Al(NO_3)_3# .The prefix poly- means many and the prefix mono- means one.
Therefore, polyatomic ions are composed of many atoms. Europa universalis iv: wealth of nations collection download for mac.
Examples include:
#NO_3^(-1)# and#PO_4^(-3)# .The nitrate ion
#NO_3^(-1)# is composed of one nitrogen atoms and three oxygen atoms arranged with an imbalance of one extra electron.The phosphate ion
#PO_4^(-3)# is composed of one phosphate ion and four oxygen atoms arranges with an imbalance of three extra electrons.Most polyatomic ions are negative with the main exceptions being
#NH_4^(+1)# and#H_3O^(+1)# . The ammonium ion which is composed of one nitrogen and 4 hydrogen with an imbalance of one less electron. The hydronium ion is created when water takes on a dissociated#H^+# ion in an acid solution.Monatomic ions are single atoms with a positive or negative charge like Calcium
#Ca^(+2)# having two less electrons and Chloride#Cl^(-1)# having one more electron.I hope this was helpful.
SMARTERTEACHER
Polyatomic Atoms Explained
Questions
